Physical Address
23,24,25 & 26, 2nd Floor, Software Technology Park India, Opp: Garware Stadium,MIDC, Chikalthana, Aurangabad, Maharashtra – 431001 India
Physical Address
23,24,25 & 26, 2nd Floor, Software Technology Park India, Opp: Garware Stadium,MIDC, Chikalthana, Aurangabad, Maharashtra – 431001 India
There is no question that Earth is warming at a rate like never before, and scientists have determined that many of the anthropogenic activities, such as the burning of fossil fuel, the release of greenhouse gases, and industrial waste, are the main contributors towards global warming over the last few centuries. It was discovered that greenhouse gases, like carbon dioxide, methane, nitrous oxides, and water vapour, cause the Earth to warm by trapping heat in the atmosphere. Scientists are now researching carbon capture techniques and concepts like liquid 3 to remove greenhouse gases from the atmosphere.
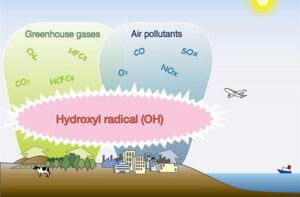
Today we are shedding light on a tiny molecule that may help mitigate climate change, known as the “detergent” of the atmosphere because they help clean up pollutants such as methane and other greenhouse gases. Scientifically it is called Hydroxyl radicals (OH).
First, let’s look into hydroxyl radicals and their functionality.
What is a Hydroxyl radical (OH)?
Hydroxyl radicals are highly reactive, short-lived molecules that play an important role in the Earth’s atmosphere. OH, is produced naturally in the atmosphere by a series of chemical reactions, mainly involving the reaction of ozone (O3) with water vapor (H2O). OH, can also be produced through the photolysis of nitrous oxide (N2O) or the reaction of sunlight with certain organic compounds.
Hydroxyl radical (OH) is the major purifying agent or the chemical scavenger in the lower atmosphere (troposphere), which serves as the main sink for many pollutants and greenhouse gases like Methane (CH4), hydrochlorofluorocarbons (HCFCs), and hydrofluorocarbons.
OH is a key player in the chemical reactions that lead to the formation of ozone (O3), which is both a greenhouse gas and a pollutant. In addition, OH reacts with methane (CH4), one of the most potent greenhouse gases, to produce water vapor (H2O) and carbon dioxide (CO2), which are less potent greenhouse gases.
OH also reacts with other atmospheric pollutants, such as nitrogen oxides (NOx) and sulfur dioxide (SO2), that can harm human health and damage ecosystems. In this way, OH helps to reduce the concentrations of these pollutants in the atmosphere.
Hydroxyl radicals are present in tiny quantities and have a very short lifespan of less than a second, but they remove about 85% of methane in the air. So, think of them as Pac-Man in the atmosphere.
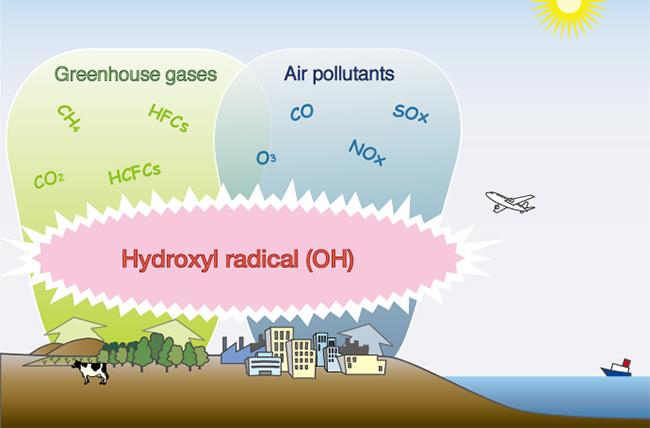
Figure 01 – Hydroxyl radical (OH): A group of reactive oxidant species that is produced by the reaction of excited atomic oxygen (O1D) with water vapour in the air.
Made up of one Hydrogen atom and one oxygen atom, Hydroxyl Radicals are highly reactive in nature because they have an unpaired electron, which tends to transfer to other molecules. For example, the short-lived OH radical (•HO) is the most important oxidant in the troposphere and lower stratosphere. It is the neutral form of the hydroxide ion (HO–). Due to this high reactivity of Hydroxyl radicals, it initiates the removal of toxic gases from the atmosphere. As a result, it significantly impacts the atmosphere’s climate, air quality, and oxidation capacity.
Hydroxyl radical concentrations remain largely stable over time. New Research considers secondary Hydroxyl radical sources, which recycle •OH after the breakdown. For example, •OH breaks down after reacting with methane and reforms in the presence of other gases. One group of gases that helps recycle Hydroxyl radicals back into the atmosphere is nitrogen oxides. (NOx)
Impact of Hydroxyl Radical on climate change
According to NASA research, a simple molecule in the atmosphere that functions as a “detergent” to break down methane and other greenhouse gases recycles itself to maintain a constant global presence in the face of rising emissions. Determining the lifetime of methane, a significant contributor to climate change, depends on understanding hydroxyl radical function in the atmosphere.
Oxidization of Methane
If a trace gas contains hydrogen atoms (such as hydrocarbons), •OH reacts by taking one hydrogen atom. For instance, with respect to methane (CH4), the most basic hydrocarbon, and •OH reacts, water and a methyl radical (•CH3) are produced.
CH4 + •OH + O2 → CH3OO + H2O
The methyl peroxy radical is formed after the methyl radical reacts with oxygen (CH3 O2). The more stable formaldehyde molecule (HCHO), which has a lifetime of about 5-8 hours in sunlight, is then formed by parallel chains of reactions.
This way, OH is the primary inhibitor of methane concentration, a potent greenhouse gas second only to carbon dioxide in its ability to raise global temperatures.
Why is cutting methane urgent?
Even though methane is found in the atmosphere for a much shorter time than carbon dioxide with hydroxyl radicals, methane traps heat much more effectively than carbon dioxide, and studies show that Methane contributes to about 30% of the rise in global temperatures.
According to research which was published in the journal Nature and the co-author Marielle Saunois of (LSCE), the rise in Methane is a main concern because it raises the possibility of a feedback loop largely independent of human control; more the methane is released, more the warming.
Researchers also examined changes in atmospheric chemistry, especially hydroxyl radicals, and methane. In order to effectively remove methane from the atmosphere in a relatively short period of time by converting it to water and CO2, it is significant to react with the hydroxyl radical. These hydroxyl radicals are henceforth responsible for removing about 85% of the methane from the atmosphere.
Nitrogen Oxide emissions and hydroxyl radical concentrations.
Can hydroxyl radical concentrations depend on other emissions? The answer is yes. It is always dependent as it is a secondary atmospheric agent, not a primary one, and emits due to a chain reaction.
•HO is formed in the atmosphere as the initial reaction involves the photolysis of O3 by solar radiation. However, there are a few other sources, and one is the photolysis of nitrous acid (HONO) and the reaction of nitrogen monoxide (NO) with the hydroperoxy radical (HO2).
Also, it involves the gas-phase reaction with NO2, and the formation of nitric acid simultaneously helps remove NO2.
NO2 + OH° → HNO3 daytime (dominant pathway)
So, the emission of NO can result in the formation of hydroxyl radicals and a decrease in methane.
The researchers discovered an interesting phenomenon during the COVID-19 lockdowns. As the global pollution levels were significantly reduced during the COVID-19 period, it caused a decrease in nitrogen oxide emissions, as the main source of nitrogen oxide emissions into the atmosphere is fossil fuel burning.
This resulted in a 1.6% decrease in hydroxyl radical concentrations in 2020 compared to the previous year, which meant that the amount of Methane removed was also reduced, giving rise to a higher concentration of Methane in the atmosphere. Studies show that a 20% decrease in nitrogen oxide could result in a two-fold increase in methane.
Hydroxyl radicals also form a wide range of secondary species, for example, ground-level ozone and secondary organic aerosol. Therefore, it is easier to build connections among atmospheric agents by considering all the factors.
Use of Climate Models to Determine Hydroxyl Radicals
Models help us to work through complicated problems and understand complex systems. They also allow us to test theories and solutions. For example, the climate is also understood with numerical models, which are quantitative methods to simulate the interactions of the essential drivers of climate change.
For example, quantifying the amount of OH present in the atmosphere is challenging as it is not directly emitted as a primary source but as a secondary source. However, researchers predict the presence of OH based on its chemical production from other “precursor” gases.
To make these predictions, researchers use computer simulations. These data are also related to climate models. Climate models describe and predict how OH and methane interact throughout the atmosphere.
On a global scale, Hydroxyl radical concentration is extremely low, ranging from 1×105 to 2×107 molecules cm 3. However, 15% of •OH reacts primarily with methane (CH4). In the reaction of OH with methane, OH is also removed and recycled. However, it’s not guaranteed that as the atmosphere continues to evolve with global climate change, OH levels will continue to recycle in the same way into the future.
Novel R & D on hydroxyl radical production, Pros and Cons.
Since the 1970s, numerous types of research have been conducted into producing hydroxyl radicals within indoor or enclosed spaces. This developed equipment requires UV energy and supplemental chemicals to be added. The latest devices produce super-charged hydroxyl radicals that can be projected into space to sanitize the air and surfaces. They can often be fitted into existing ventilation systems or standalone units within the space.
Despite its essential role in the atmosphere, the impact of OH on global climate change is complex and still needs to be fully understood. OH, concentrations can be affected by a variety of factors, including temperature, humidity, and the attention of other chemicals in the atmosphere.
Some studies suggest that changes in OH concentrations could have a significant impact on the Earth’s climate. However, other studies suggest that the impact of OH on climate change may be limited because OH concentrations are already high enough to remove most pollutants from the atmosphere effectively. In addition, changes in OH concentrations could also affect the concentrations of other chemicals in the atmosphere, which could have complex and unpredictable effects on the Earth’s climate.
It is essential to understand how and why OH may change in the future and become paramount for predicting changes in the climate. However, future models’ projections strongly disagree with how OH responds to changing emissions and climate. However, this method of Climate Change mitigation is not in practice since there is much more research to be carried out for its proper implementation.
Modern technologies to reverse climate change.
Other than current strategies against the climate crisis, some new concepts and technologies are predicted by scientists similar to hydroxyl radicals. These concepts will investigate radical approaches with new technologies because of fears that current systems will not be sufficient and can help to continue irreversible damage to the planet.
Cambridge-based researchers intend to establish a research hub to create fresh approaches to climate change mitigation. Some strategies include refreezing the Earth’s poles, removing CO2 from the atmosphere, and recycling CO2.
The idea behind the Refreezing the Poles theory is to pump seawater through excellent nozzles up tall masts on crewless ships to “brighten” the clouds above the poles and refreeze them. This results in the production of minute salt particles injected into the clouds, increasing their size and reflectiveness and cooling the areas beneath them.
A variation of the concept of carbon capture and storage is recycling CO2 (CCS). CCS involves gathering carbon dioxide emissions from steel plants and coal- or gas-fired power plants and burying them underground. Additionally, some plans call for fertilizing the sea with iron salts to encourage plankton growth. We can look forward to better alternatives to combat climate change in the future.
Conclusion
Hydroxyl radicals (OH) are highly reactive and short-lived molecules that play an important role in the Earth’s atmosphere because they help to clean up pollutants such as methane and other greenhouse gases.
Most of the troposphere’s trace gases are oxidized by •OH into water-soluble byproducts removed by precipitation and snowfall. Only a tiny number of tropospheric compounds do not react with the hydroxyl radical at all or only very slowly. These include carbon dioxide, nitrous oxide, and chlorofluorocarbons (CFCs).
On a global scale, the concentration of Hydroxyl radicals is extremely low, ranging from 1×105 to 2×107 molecules cm-3. Around 30% of the OH is removed from the atmosphere in reactions with organic compounds, and 15% reacts with methane (CH4). Therefore, these hydroxyl radicals are responsible for releasing about 85% of the methane from the atmosphere and helping to mitigate climate change.
Hydroxyl radicals also have drawbacks, but we can be hopeful about the impact of hydroxyl radicals on climate change mitigation with more developed experiments and enhanced technologies. While OH plays an important role in the Earth’s atmosphere, its impact on climate change mitigation is still an area of active research and debate.
-With inputs from Nuwandhara Mudalige –